Abstract
Background:
Despite significant improvements in the prognosis of chronic myeloid leukemia (CML) achieved by targeted therapy with tyrosine kinase inhibitors (TKIs), a small proportion of cases may not respond to TKIs or may relapse after an initial response, and then progress from chronic phase (CP) to blastic crisis (BC), characterized by a dismal prognosis. It remained uncertain whether the genetic lesions in addition to the BCR-ABL1 fusion could predict clinical outcomes of CML in the TKI era.
Aim:
To study the mutational profiles at each stage of CML and the prognostic significance of somatic mutations in addition to the BCR-ABL1 fusion in the TKI era.
Patients and Methods:
We performed targeted sequencing in 81 CML patients chosen retrospectively. 10 patients had optimal response to TKIs by European LeukemiaNet criteria and maintained durable major molecular response more than 5 years. 71 patients had progressed to accelerated phase (AP) or BC, of whom 43 had sequencing performed at paired CP and AP/BC samples, 28 at AP or BC samples. Totally, we analyzed 53 CP, 20 AP, and 61 BC samples. The targeted resequencing gene panel, covering 386 genes which were recurrently mutated in hematologic malignancies, were performed on a HiSeq 4000 NGS platform (Illumina).
Results:
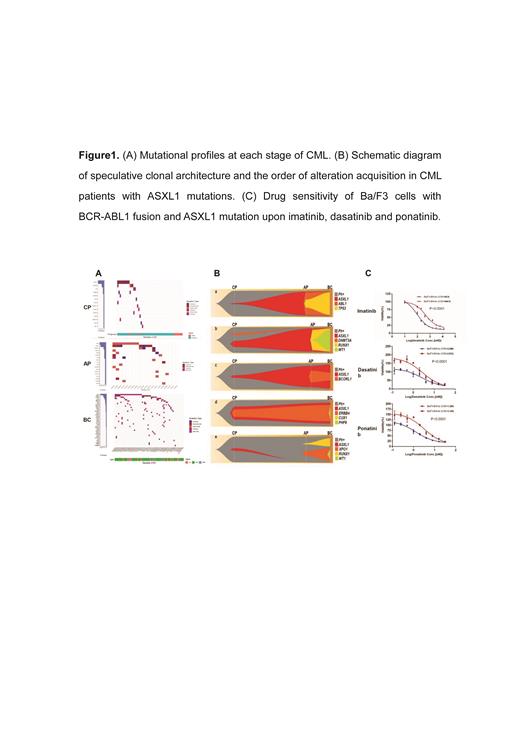
Among the 53 CP samples, 20 (37.7%) had mutations involving 14 genes, and the number of mutated genes in each patient was 0-3 (median 0). ASXL1 was the most commonly mutated gene, 10/53 (18.9%) patients had this mutation, followed by KMT2D (4/53, 7.5%), PC (2/53, 3.8%), ERBB4 (2/53, 3.8%). ASXL1 mutation mainly existed in 43 patients with progressed disease , while only one case carried this mutation in 10 patients responsive to TKIs (20.9% vs 10%). 17/20 (85%) AP samples (including 10 patients progressed to AP and the other 10 patients who eventually progressed to BC from AP ) carried mutations involving 18 genes, the number of mutated genes in each patient was 0-6 (median 1.5). ABL1 was the most commonly mutated gene, and 8/20 (40%) patients had this mutation. The second was the ASXL1 mutation, 7 (7/20, 35%) patients carried this mutation. The other genes mutated in more than 2 patients included BCORL1 (3/20, 15%), RUNX1 (2/20, 10%), PHF6 (2/20, 10%), KMT2D (2/20, 10%), ATM (2/20, 10%). 54/61 (88.5%) BC samples (44 with myeloid crisis, 14 with lymphoid crisis, 3 with mixed phenotypic crisis) carried mutations, involving 41 genes, and the number of mutated genes in each patient was 0-9 (median 2). Similar to the mutation status in AP, the most commonly mutated gene was also ABL1, 24/61 (39.3%) patients carried this gene mutation, followed by ASXL1 mutation (13/61, 21.3%), and the other genes were in order, RUNX1 (11/61, 18.0%), WT1 (8/61, 13.1%), GATA2 (6/61, 9.8%), MED12 (5/61, 8.2%), IDH1 (5/61, 8.2%), TP53 (4/61 , 6.6%), KMT2D (4/61, 6.6%), etc. (Figure 1A)
Among all the samples, 34 nonsynonymous variants in the ASXL1 gene were identified in 31 samples of 21 patients ( 3 samples with two variants). All the variants were frameshift and nonsense mutations, localized at the last exon of the ASXL1 gene. 13/21 patients with ASXL1 mutations had multi-stage samples. The median VAF of the ASXL1 mutations in the advanced stage was 31.4% (0-47.0%), which was significantly higher than that in CP at diagnosis (7.0%, 0-27.2%, P=0.033). Most of the ASXL1 mutations detected in CP expanded at the advanced disease, and were accompanied with other additional gene abnormalities, such as ABL1, RUNX1 and WT1 mutations, with the VAF similar to or lower than that of the ASXL1 mutations. In a few cases, the ASXL1 mutant clones in the CP disappeared, suggesting that some ASXL1 mutations may be clonal hematopoiesis unrelated to disease progression.(Figure 1B)
In order to evaluate the effects of ASXL1 mutations on sensitivity to TKIs in vitro. We co-expressed P210-BCR-ABL1 fusion and ASXL1 mutation (G646Wfs*12) in Ba/F3 cells. Compared to Ba/F3 cells co-expressing BCR-ABL1 fusion and ASXL1 mutation (Ba/F3-BA/As), Ba/F3-BCR-ABL1 cells without ASXL1 mutation (Ba/F3-BA/Ve) showed higher sensitivity to TKIs, including imatinib, dasatinib and nilotinib.(Figure 1C)
Conclusions:
These results demonstrated the genetic lesions accumulated during the progression of CML from CP to BC. ASXL1 mutations were the most common genetic lesion in CP at diagnosis and may confer a poor prognosis, as it reduced the sensitivity to TKIs.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal